Product Name :
CD140a Recombinant Protein Swiss-Prot :
P16234 Host :
E.coli Tag :
≥0.5mg/ml Amino acid Sequence :
QLSLPSILPNENEKVVQLNSSFSLRCFGESEVSWQYPMSEEESSDVEIRNEENNSGLFVTVLEVSSASAAHTGLYTCYYNHTQTEENELEGRHIYIYVPDPDVAFVPLGMTDYLVIVEDDDSAIIPCRTTDPETPVTLHNSEGVVPASYDSRQGFNGTFTVGPYICEATVKGKKFQTIPFNVYALKATSELDLEMEALKTVYKSGETIVVTCAVFNNEVVDLQWTYPGEVKGKGITMLEEIKVPSIKLVYTLTVPEATVKDSGDYECAARQATREVKEMKKVTISVHEKGFIEIKPTFSQLEAVNLHEVKHFVVEVRAYPPPRISWLKNNLTLIENLTEITTDVEKIQEIRYRSKLKLIRAKEEDSGHYTIVAQNEDAVKSYTFELLTQVPSSILDLVDDHHGSTGGQTVRCTAEGTPLPDIEWMICKDIKKCNNETSWTILANNVSNIITEIHSRDRSTVEGRVTFAKVEETIAVRCLAKNLLGAENRELKLVAPTLRSELTVA Restriction sites :
NdeI-XhoI Background :
Platelet derived growth factor (PDGF) family proteins exist as several disulphide-bonded, dimeric isoforms (PDGF AA, PDGF AB, PDGF BB, PDGF CC, and PDGF DD) that bind in a specific pattern to two closely related receptor tyrosine kinases, PDGF receptor α (PDGFRα) and PDGF receptor β (PDGFRβ). PDGFRα and PDGFRβ share 75% to 85% sequence homology between their two intracellular kinase domains, while the kinase insert and carboxy-terminal tail regions display a lower level (27% to 28%) of homology. PDGFRα homodimers bind all PDGF isoforms except those containing PDGF D. PDGFRβ homodimers bind PDGF BB and DD isoforms, as well as the PDGF AB heterodimer. The heteromeric PDGF receptor α/β binds PDGF B, C, and D homodimers, as well as the PDGF AB heterodimer. PDGFRα and PDGFRβ can each form heterodimers with EGFR, which is also activated by PDGF. Various cells differ in the total number of receptors present and in the receptor subunit composition, which may account for responsive differences among cell types to PDGF binding. Ligand binding induces receptor dimerization and autophosphorylation, followed by binding and activation of cytoplasmic SH2 domain-containing signal transduction molecules, such as GRB2, Src, GAP, PI3 kinase, PLCγ, and NCK. A number of different signaling pathways are initiated by activated PDGF receptors and lead to control of cell growth, actin reorganization, migration, and differentiation. Tyr751 in the kinase-insert region of PDGFRβ is the docking site for PI3 kinase. Phosphorylated pentapeptides derived from Tyr751 of PDGFRβ (pTyr751-Val-Pro-Met-Leu) inhibit the association of the carboxy-terminal SH2 domain of the p85 subunit of PI3 kinase with PDGFR. Tyr740 is also required for PDGFRβ-mediated PI3 kinase activation. Soluble :
PBS, 4M Urea, PH7.4 Purification&Purity :
Transferred into competent cells and the supernatant was purified by NI column affinity chromatography and the purity is > 85% (by SDS-PAGE). Storage&Stability :
Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze-thaw cycles. Expression vector :
pet-22b(+) BiowMW :
~56kDa Note :
For research use only, not for use in diagnostic procedure. concentration :
≥0.5mg/ml
CD140a Recombinant Protein Swiss-Prot :
P16234 Host :
E.coli Tag :
≥0.5mg/ml Amino acid Sequence :
QLSLPSILPNENEKVVQLNSSFSLRCFGESEVSWQYPMSEEESSDVEIRNEENNSGLFVTVLEVSSASAAHTGLYTCYYNHTQTEENELEGRHIYIYVPDPDVAFVPLGMTDYLVIVEDDDSAIIPCRTTDPETPVTLHNSEGVVPASYDSRQGFNGTFTVGPYICEATVKGKKFQTIPFNVYALKATSELDLEMEALKTVYKSGETIVVTCAVFNNEVVDLQWTYPGEVKGKGITMLEEIKVPSIKLVYTLTVPEATVKDSGDYECAARQATREVKEMKKVTISVHEKGFIEIKPTFSQLEAVNLHEVKHFVVEVRAYPPPRISWLKNNLTLIENLTEITTDVEKIQEIRYRSKLKLIRAKEEDSGHYTIVAQNEDAVKSYTFELLTQVPSSILDLVDDHHGSTGGQTVRCTAEGTPLPDIEWMICKDIKKCNNETSWTILANNVSNIITEIHSRDRSTVEGRVTFAKVEETIAVRCLAKNLLGAENRELKLVAPTLRSELTVA Restriction sites :
NdeI-XhoI Background :
Platelet derived growth factor (PDGF) family proteins exist as several disulphide-bonded, dimeric isoforms (PDGF AA, PDGF AB, PDGF BB, PDGF CC, and PDGF DD) that bind in a specific pattern to two closely related receptor tyrosine kinases, PDGF receptor α (PDGFRα) and PDGF receptor β (PDGFRβ). PDGFRα and PDGFRβ share 75% to 85% sequence homology between their two intracellular kinase domains, while the kinase insert and carboxy-terminal tail regions display a lower level (27% to 28%) of homology. PDGFRα homodimers bind all PDGF isoforms except those containing PDGF D. PDGFRβ homodimers bind PDGF BB and DD isoforms, as well as the PDGF AB heterodimer. The heteromeric PDGF receptor α/β binds PDGF B, C, and D homodimers, as well as the PDGF AB heterodimer. PDGFRα and PDGFRβ can each form heterodimers with EGFR, which is also activated by PDGF. Various cells differ in the total number of receptors present and in the receptor subunit composition, which may account for responsive differences among cell types to PDGF binding. Ligand binding induces receptor dimerization and autophosphorylation, followed by binding and activation of cytoplasmic SH2 domain-containing signal transduction molecules, such as GRB2, Src, GAP, PI3 kinase, PLCγ, and NCK. A number of different signaling pathways are initiated by activated PDGF receptors and lead to control of cell growth, actin reorganization, migration, and differentiation. Tyr751 in the kinase-insert region of PDGFRβ is the docking site for PI3 kinase. Phosphorylated pentapeptides derived from Tyr751 of PDGFRβ (pTyr751-Val-Pro-Met-Leu) inhibit the association of the carboxy-terminal SH2 domain of the p85 subunit of PI3 kinase with PDGFR. Tyr740 is also required for PDGFRβ-mediated PI3 kinase activation. Soluble :
PBS, 4M Urea, PH7.4 Purification&Purity :
Transferred into competent cells and the supernatant was purified by NI column affinity chromatography and the purity is > 85% (by SDS-PAGE). Storage&Stability :
Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze-thaw cycles. Expression vector :
pet-22b(+) BiowMW :
~56kDa Note :
For research use only, not for use in diagnostic procedure. concentration :
≥0.5mg/ml
Blocking peptide available as NCP0265P

 CD140a Recombinant Protein
CD140a Recombinant Protein 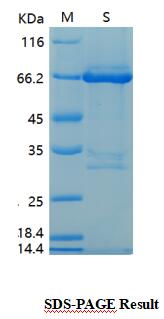
 Datasheet
Datasheet COA
COA MSDS
MSDS SHIP
SHIP