Product Name :
β-Actin Recombinant Protein Swiss-Prot :
P60709 Host :
E.coli Tag :
Amino acid Sequence :
MDDDIAALVVDNGSGMCKAGFAGDDAPRAVFPSIVGRPRHQGVMVGMGQKDSYVGDEAQSKRGILTLKYPIEHGIVTNWDDMEKIWHHTFYNELRVAPEEHPVLLTEAPLNPKANREKMTQIMFETFNTPAMYVAIQAVLSLYASGRTTGIVMDSGDGVTHTVPIYEGYALPHAILRLDLAGRDLTDYLMKILTERGYSFTTTAEREIVRDIKEKLCYVALDFEQEMATAASSSSLEKSYELPDGQVITIGNERFRCPEALFQPSFLGMESCGIHETTFNSIMKCDVDIRKDLYANTVLSGGTTMYPGIADRMQKEITALAPSTMKIKIIAPPERKYSVWIGGSILASLSTFQQMWISKQEYDESGPSIVHRKCF Restriction sites :
NdeI-XhoI Background :
Actin, a ubiquitous eukaryotic protein, is the major component of the cytoskeleton. At least six isoforms are known in mammals. Nonmuscle β- and γ-actin, also known as cytoplasmic actin, are ubiquitously expressed, controlling cell structure and motility. While all actin isoforms are highly homologous, cytoplasmic β- and γ-actin protein sequences differ by only four biochemically similar amino acids. For this reason, antibodies raised to β-actin may cross-react with γ-actin, and vice versa. α-cardiac and α-skeletal actin are expressed in striated cardiac and skeletal muscles, respectively; two smooth muscle actins, α- and γ-actin, are found primarily in vascular smooth muscle and enteric smooth muscle, respectively. These actin isoforms regulate the contractile potential of muscle cells. Actin exists mainly as a fibrous polymer, F-actin. In response to cytoskeletal reorganizing signals during processes such as cytokinesis, endocytosis, or stress, cofilin promotes fragmentation and depolymerization of F-actin, resulting in an increase in the monomeric globular form, G-actin. The ARP2/3 complex stabilizes F-actin fragments and promotes formation of new actin filaments. Research studies have shown that actin is hyperphosphorylated in primary breast tumors. Cleavage of actin under apoptotic conditions has been observed in vitro and in cardiac and skeletal muscle, as shown in research studies. Actin cleavage by caspase-3 may accelerate ubiquitin/proteasome-dependent muscle proteolysis. Soluble :
PBS, PH7.4 Purification&Purity :
Transferred into competent cells and the supernatant was purified by NI column affinity chromatography and the purity is > 85% (by SDS-PAGE). Storage&Stability :
Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze-thaw cycles. Expression vector :
pet-22b(+) BiowMW :
~43kDa Note :
For research use only, not for use in diagnostic procedure. concentration :
β-Actin Recombinant Protein Swiss-Prot :
P60709 Host :
E.coli Tag :
Amino acid Sequence :
MDDDIAALVVDNGSGMCKAGFAGDDAPRAVFPSIVGRPRHQGVMVGMGQKDSYVGDEAQSKRGILTLKYPIEHGIVTNWDDMEKIWHHTFYNELRVAPEEHPVLLTEAPLNPKANREKMTQIMFETFNTPAMYVAIQAVLSLYASGRTTGIVMDSGDGVTHTVPIYEGYALPHAILRLDLAGRDLTDYLMKILTERGYSFTTTAEREIVRDIKEKLCYVALDFEQEMATAASSSSLEKSYELPDGQVITIGNERFRCPEALFQPSFLGMESCGIHETTFNSIMKCDVDIRKDLYANTVLSGGTTMYPGIADRMQKEITALAPSTMKIKIIAPPERKYSVWIGGSILASLSTFQQMWISKQEYDESGPSIVHRKCF Restriction sites :
NdeI-XhoI Background :
Actin, a ubiquitous eukaryotic protein, is the major component of the cytoskeleton. At least six isoforms are known in mammals. Nonmuscle β- and γ-actin, also known as cytoplasmic actin, are ubiquitously expressed, controlling cell structure and motility. While all actin isoforms are highly homologous, cytoplasmic β- and γ-actin protein sequences differ by only four biochemically similar amino acids. For this reason, antibodies raised to β-actin may cross-react with γ-actin, and vice versa. α-cardiac and α-skeletal actin are expressed in striated cardiac and skeletal muscles, respectively; two smooth muscle actins, α- and γ-actin, are found primarily in vascular smooth muscle and enteric smooth muscle, respectively. These actin isoforms regulate the contractile potential of muscle cells. Actin exists mainly as a fibrous polymer, F-actin. In response to cytoskeletal reorganizing signals during processes such as cytokinesis, endocytosis, or stress, cofilin promotes fragmentation and depolymerization of F-actin, resulting in an increase in the monomeric globular form, G-actin. The ARP2/3 complex stabilizes F-actin fragments and promotes formation of new actin filaments. Research studies have shown that actin is hyperphosphorylated in primary breast tumors. Cleavage of actin under apoptotic conditions has been observed in vitro and in cardiac and skeletal muscle, as shown in research studies. Actin cleavage by caspase-3 may accelerate ubiquitin/proteasome-dependent muscle proteolysis. Soluble :
PBS, PH7.4 Purification&Purity :
Transferred into competent cells and the supernatant was purified by NI column affinity chromatography and the purity is > 85% (by SDS-PAGE). Storage&Stability :
Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze-thaw cycles. Expression vector :
pet-22b(+) BiowMW :
~43kDa Note :
For research use only, not for use in diagnostic procedure. concentration :
Blocking peptide available as NCP0397P

 β-Actin Recombinant Protein
β-Actin Recombinant Protein 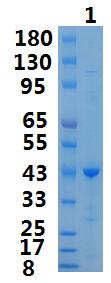
 Datasheet
Datasheet COA
COA MSDS
MSDS SHIP
SHIP